Panther Fusion® GI Bacterial Assay
A fully-automated approach to gastrointestinal (GI) molecular testing of Salmonella, Campylobacter, Shigella/Enteroinvasive Escherichia coli (EIEC) and Shigatoxigenic Escherichia coli (STEC).1,2

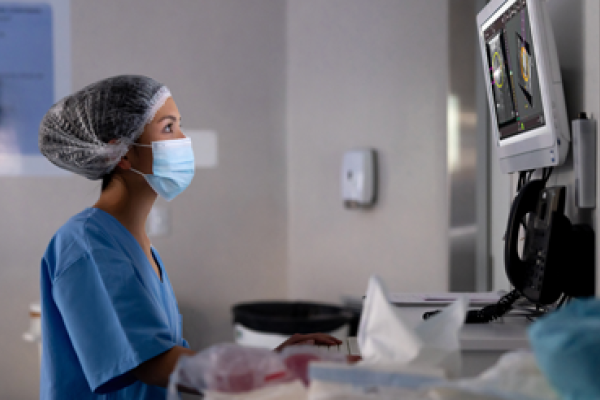
Accuracy & Flexibility for the Patient & the Lab
With highly sensitive and reliable results,1,2 the Panther Fusion GI Bacterial assay supports informed clinical decision-making while seamlessly integrating into existing laboratory workflows.
A New Approach to Gastrointestinal Bacterial Testing
Acute diarrhoea is a leading cause of outpatient visits, hospitalisation, and lost quality of life.1
Act Fast
Trust more than just instinct. Panther Fusion GI Bacterial assay delivers the speed and sensitivity to diagnose enteric disease rapidly.2
Accurate
Leverage on accurate diagnostics to guide treatment decisions.1
Relevant
Tailor results2 to deliver what matters to you and your patients.

Simplify & Scale the Future of Diagnostics
The Panther Fusion GI Bacterial Assay is part of Panther® Scalable Solutions, a portfolio combining a broad, high performing assay menu with high throughput automation. Designed to flexibly scale to meet your needs, from a single patient result to population-level screening.
Proven Performance
Providing high clinical performance and accuracy in gastrointestinal bacterial testing.1 Panther Fusion GI Bacterial assay has a very high Positive Percentage Agreement (PPA) and Negative Percentage Agreement (NPA) with clinical comparator, in retrospective samples.1
100% PPA
99% NPA
Salmonella spp.
100% PPA
99% NPA
Campylobacter spp.
100% PPA
99.5% NPA
Shigella/EIEC
100% PPA
95.6% NPA
Shiga Toxins 1 and 2
Evidence. Insight. Collaboration.
Our education portal improves patient care through excellence in education, communication of clinical and scientific evidence, and partnerships with the healthcare community.