Aptima® SARS-CoV-2/Flu Assay
Fully automated, high-throughput assay for the detection of SARS-CoV-2, influenza A (Flu A) and influenza B (Flu B) in one single assay.1

The Performance & Flexibility you Need
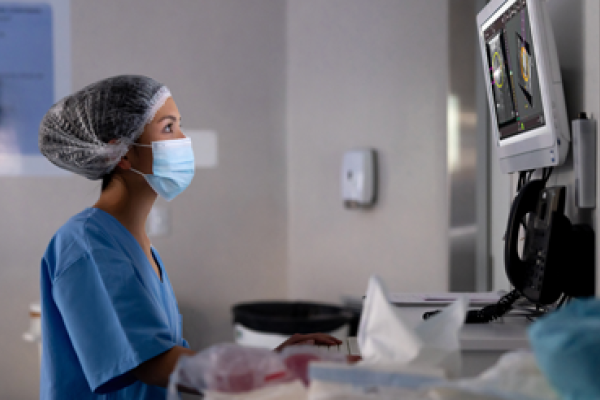
Accurate and fully automated testing is critical in the fight against the threat of SARS-CoV-2. This is key to quickly identifying who’s infected and subsequently helping alleviate the spread of this virus. The assay is used for the qualitative detection and differentiation of SARS-CoV-2, influenza A virus and influenza B virus from individuals suspected of respiratory viral infection consistent with COVID-19.1
The Power to Choose & Potential to Grow
Leverage the flexibility and scalability of the Panther® System.
Efficiency
Meet any urgent need for high-throughput and fully-automated testing, delivering more than 1000 test results in 24 hours.*,2
Accuracy
Detect and differentiate SARS-CoV-2 and influenzas A and B during respiratory season to guide patient management.1
Variety
The assays can be run alongside current infectious disease, women’s health and virology assays, enabling laboratories to unlock the free capacity on their existing Panther Systems.
Simplify & Scale the Future of Diagnostics
The Aptima SARS-CoV-2 /Flu Assay is part of Panther® Scalable Solutions, a portfolio combining a broad, high performing assay menu with high throughput automation. Designed to flexibly scale to meet your needs, from a single patient result to population-level screening.

Excellent Clinical Performance
Evaluated in comparison to the Aptima SARS-CoV-2 Assay and the Panther Fusion Flu A/B/RSV assay, using a panel of remnant clinical nasopharyngeal specimens collected from patients with signs and symptoms of respiratory infection.
96.1% Positive agreement
99.6% Negative agreement SARS-CoV-21
100% Positive agreement
99.2% Negative agreement Influenza A1
100% Positive agreement
100% Negative agreement Influenza B1

Applicable Specimen Types
Applicable specimen types include nasopharyngeal and nasal swab specimens collected in UTM/VTM, saline, Liquid Amies or Aptima Specimen Transport Medium.1
Evidence. Insight. Collaboration.
Our education portal improves patient care through excellence in education, communication of clinical and scientific evidence, and partnerships with the healthcare community.
Insights
*The number of actual test results per day may vary based on individual lab practices and workflows
Aptima SARS-CoV-2/Flu Assay [package insert]. AW-22604-001 Rev. 004. San Diego, CA: Hologic, Inc.; 2023
Panther/Panther Fusion System Operator´s Manual. AW-26055-001 Rev 001, San Diego, CA: Hologic Inc.; 2022.